Updated: 2019-10-30
To fully reflect the monitoring of adverse events in China's medical devices in 2018, the National Center for Adverse Drug Reaction Monitoring has compiled and released on October 30, 2019 the Annual Report for National Medical Device Adverse Event Monitoring (2018), which is excerpted as follows:
I. Progress of medical device adverse events monitoring
In 2018, with the pioneering efforts of our staff, the national medical device adverse event monitoring has continued to advance, the monitoring scope continued to expand, the report collection capability was significantly improved, and the number of reports saw a persistent increase. The ability of monitoring personnel to analyze and evaluate adverse events has been continuously improved to effectively detect and deal with risks, the principal responsibility of manufacturers' monitoring of adverse events has been gradually implemented, and new progress has been made in this field.
(I) The construction of monitoring information system has been promoted, and network coverage has been expanded to improve data quality
In 2018, the Information System for National Medical Device Adverse Event Monitoring has received more than 400,000 Reports for Suspected Adverse Events of Medical Devices, and the quality of the reports was improved. There are more than 270,000 registered users at the grassroots level of the system, of which more than 13,000 are manufacturers, an increase of 16.44% over last year. 95.9% of districts and counties across China reported medical device adverse events, with an average of 305 reports per million people. In addition, the National Center for Adverse Drug Reaction Monitoring completed the establishment of a new information system for monitoring adverse events in medical devices, realizing the replacement of the online reporting system for such events in China, provide strong support for the implementation of the Administrative Measures for Monitoring and Re-evaluating the Adverse Events of Medical Devices (hereinafter referred to as the Measures) .
(II) The analysis and evaluation of monitoring data are reinforced for product risks mining and promoting the safety of medical devices.
In 2018, the evaluation and disposal of risk signals for the monitoring of adverse events in medical devices were carried out in depth. We've strengthened the daily monitoring, early warning analysis and quarterly summary of national medical device adverse event reports. Based on the risk conditions, 3 issues of Medical Device Adverse Event Information Notification and 6 Medical Device Pharmacovigilance Expresses were issued throughout the year. The intensive monitoring of adverse events in medical devices 13th Five-Year Plan continued to advance. The National Center for ADR Monitoring has organized inspections on key surveillance work. The relevant undertaking units proactively collected monitoring data to dig deeper into the risks of medical devices, and the relevant work saw steady progress.
(III) We carried out technical training on monitoring regulations, and actively participated in international exchanges to improve the level of the monitoring teams
To support the implementation of the newly revised Measures, the National Adverse Drug Reaction Monitoring Center trained a total of 393 monitoring institutions throughout the year, achieving full coverage of training at provinciallevel monitoring institutions. Supervision departments at all levels have organized special training courses on the Measures and related guiding principles to improve the ability of monitoring personnel and reinforce the principal responsibility of production enterprises. In addition, we actively followed up the progress of adverse event terminology in IMDRF (International Medical Device Regulators Forum) and promoted China's participation in the National Competent Authority Report (NCAR) System, which further enhanced the level of internationalization.
II. General situation of medical device adverse event reporting
(I) Overview of reporting in 2018
1. Number of Medical Device Adverse Event Reports in China
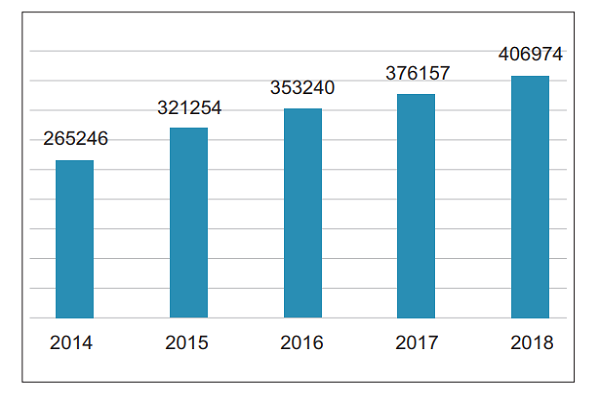
In 2018, the National Medical Device Adverse Event Monitoring Information System received a total of 406,974 reports of suspected medical device adverse events, an increase of 8.19% over 2017, reflecting the increasing awareness of China's medical device adverse event reporting and the effective enhancement of report collection capabilities (Figure 1) .
Figure 1. Number of Suspected Medical Device Adverse Events Reported from 2014 to 2018
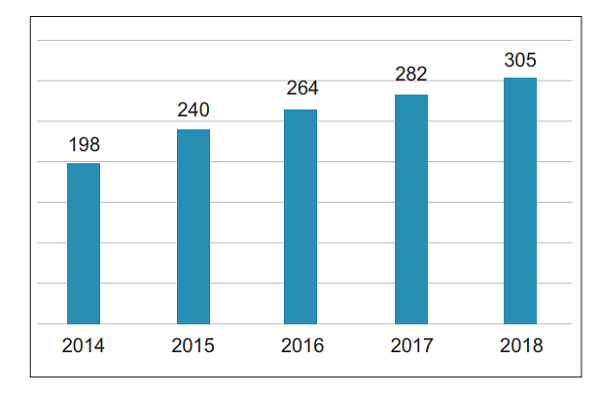
2. Average number of reports per million population In 2018, the average number of suspected medical device adverse events reported per million people in China was 305, an increase of 23 compared with 2017 (Figure 2)
Figure 2 Comparison of the number of suspected medical device adverse events reported per million people across China from 2014 to 2018
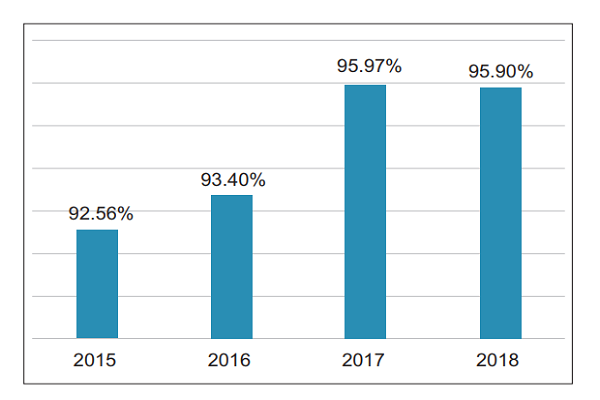
3. County-level coverage In 2018, the county-level coverage of suspected medical device adverse events in China was 95.9% (Figure 3) . (II) Number of registered grassroots users nationwide As of December 31, 2018, there were a total of 275,715 grassroots user units (including manufacturing enterprises, operating companies and users) registered in the National Medical Device Adverse Event
Figure 3 County-level coverage of suspected medical device adverse event reports from 2015 to 2018 in China
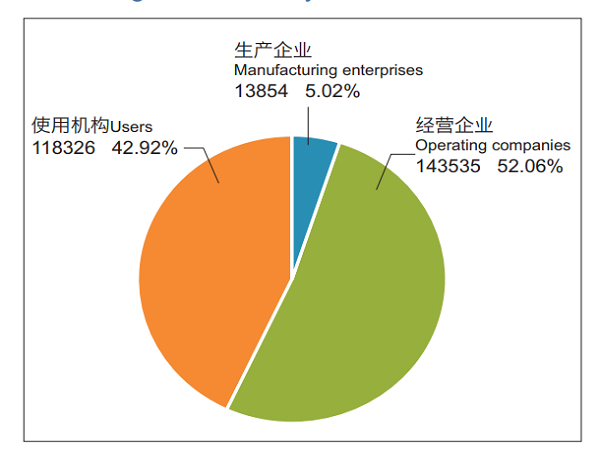
Monitoring Information System, cover 13,854 manufacturers (5.02%); 143,535 distributors (52.06%); 118,326 user units (42.92%) (Figure 4) .
Figure 4 Status of registered grassroots users in the National Medical Device Adverse Event Monitoring Information System in 2018
In 2018, the total number of registered grassroots users increased by 8.87% over 2017. It shows that the scope of monitoring of adverse events of medical devices in China has been expanding. Among them, the registered grassroots users of manufacturers, distributors and user units have increased by 16.44%, 11.59% and 4.97% over 2017, respectively (Figure 5) .
III. Statistical analysis of national medical device adverse event reports (omitted)
IV. Release of medical device adverse event information notification (omitted)
V. Issuance of Pharmacovigilance Expresses on Medical Devices (omitted)
VI. Explanation of relevant information(omitted)