On March 17, 2020, NMPA issued the2019 Annual Report for Medical Device Registration, which covers five parts: medical device registration status, medicaldevice registration application acceptance status, medical device registration approval status, innovative medical device registration approval status, and other registrationmanagement status.
Acceptance of medical device registration applications
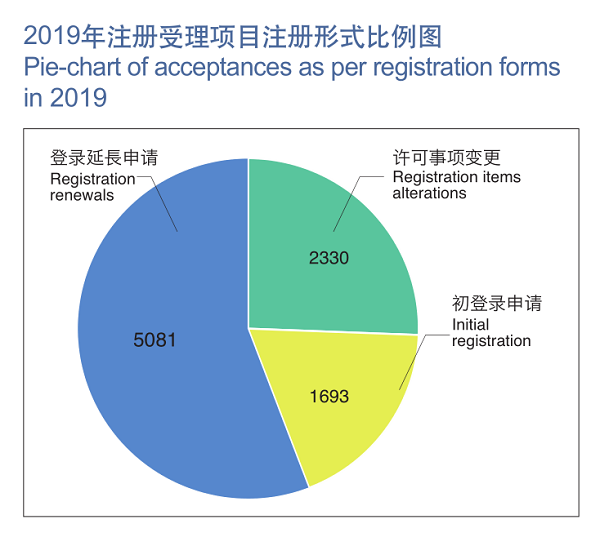
In 2019, NMPA has, as per its powers and duties, accepted a total of 9,104 applications for initial registration, registration renewals, and registration application for amendments of licensing matters of medical devices, a 37.8% increase as compared with that of 2018.
Medical Device Registration Approval
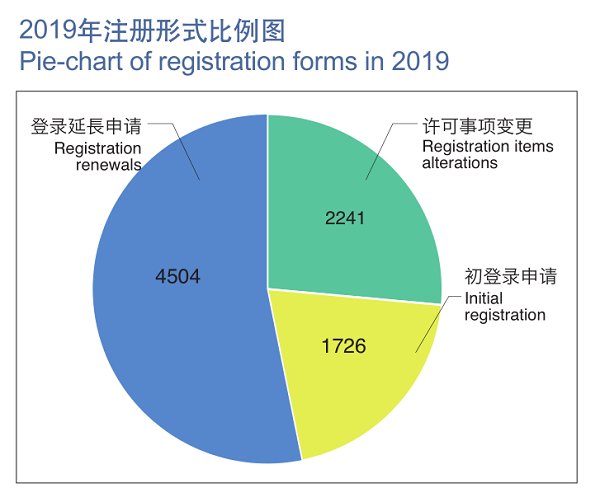
In 2019, NMPA approved a grand sum of 8,471 registrations applications, up by 53.2% YOY, covering initial registrations (1,726), registration renewals (4,504), and registration alterations (2,241) of medical devices.
Note: The statistics period of this report is from January 1, 2019 to December 31, 2019.